In situ growth of Fe3O4 on montmorillonite surface and its removal of anionic pollutants
Abstract
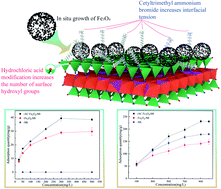
An environmentally functional material for the efficient removal of anionic pollutants in water was prepared for our study. Montmorillonite (M) was modified by hydrochloric acid (HCl) and cetyltrimethylammonium bromide (CTAB). Fe3O4 was grown in situ to prepare modified Fe3O4/montmorillonite (AC Fe3O4–Mt) composites. The number of hydroxyl sites on the surface of Mt and the surface tension were increased by HCl and CTAB modification, respectively, which enabled higher Fe3O4 surface growth, promoting the multi-directional crystallisation of Fe3O4 and improving reactivity. The XRD results show that Fe3O4 grows on the surface of Mt and has good crystallinity and high purity, meaning that AC Fe3O4–Mt is more reactive than Fe3O4. When AC Fe3O4–Mt is used to remove anionic pollutants in water, the removal effect under the synergistic action of adsorption-redox is significantly improved, and the maximum removable quantities of Cr(VI) and 2-4-dichlorophenol can reach 41.57 mg g−1 and 239.33 mg g−1, respectively. AC Fe3O4–Mt is a high efficiency and environmentally functional material with green environmental protection, which can be used in the field of sewage treatment.


 Please wait while we load your content...
Please wait while we load your content...