Vinylene-bridged donor–acceptor type porous organic polymers for enhanced photocatalysis of amine oxidative coupling reactions under visible light†
Abstract
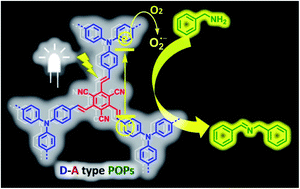
Porous organic polymers (POPs), owing to their abundant porosity, high stability and well-tunable properties, are promising candidates as heterogeneous photocatalysts for organic transformations. Here we report two vinylene-bridged donor–acceptor (D–A) structural POPs (TpTc-POP and TbTc-POP) that are facilely constructed by the electron-rich triarylamine and electron-deficient tricyanomesitylene as key building blocks by the organic base catalyzed Knoevenagel condensation. Both TpTc-POP and TbTc-POP possess hierarchical meso- and micro-pores with a high surface area. Furthermore, the unsubstituted vinylene linkages of D–A moieties in their polymer backbones extend their π-conjugation and render their broad absorption range in the visible-light region. Thus, these DA-POPs exhibited highly effective photocatalytic activities for aerobic oxidative coupling of amines to imines under visible light irradiation. This study shows the great potential of conjugated POPs with a D–A structural feature in designing highly efficient and active heterogeneous photocatalytic systems.


 Please wait while we load your content...
Please wait while we load your content...