Ribose conversion with amino acids into pyrraline platform chemicals – expeditious synthesis of diverse pyrrole-fused alkaloid compounds†
Abstract
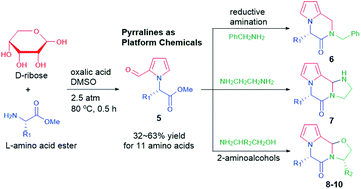
One-pot conversion of sustainable D-ribose with L-amino acid, methyl esters produced pyrrole-2-carbaldehydes 5 in reasonable yields (32–63%) under pressurized conditions of 2.5 atm at 80 °C. The value-added pyrraline compounds 5 as platform chemicals were utilized for quick installation of poly-heterocyclic cores for the development of pyrrole-motif natural and artificial therapeutic agents. A pyrrole-fused piperazin-2-one scaffold 6 was prepared by reductive amination of pyrralines 5 with benzylamine. While further cyclization of pyrralines 5 with ethane-1,2-diamine produced pyrrolo-piperazin-2-ones 7 with an extra imidazolidine ring, the reaction with 2-amino alcohols derived from natural L-amino acids, alanine, valine, and phenylalanine, respectively provided pyrrolo-piperazin-2-ones 8, 9, and 10 with oxazolidine as the third structural core. Cell viability and an anti-inflammatory effect of the synthesized compounds were briefly tested by the MTT method and the Griess assay, among which 8h and 10g exhibited significant anti-inflammatory effects with negligible cell toxicity.


 Please wait while we load your content...
Please wait while we load your content...