Synthesized akhtenskites remove ammonium and manganese from aqueous solution: removal mechanism and the effect of structural cations†
Abstract
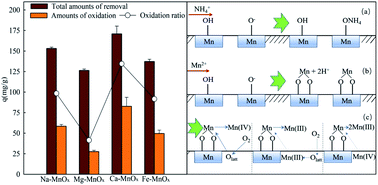
Ammonium and manganese removal by tunnel-structured manganese oxide is still enigmatic. Herein, tunnel-structured akhtenskites with different structural cations (Na–MnOx, Mg–MnOx Ca–MnOx, Fe–MnOx) were synthesized by the KMnO4 and Mn2+ reaction in the presence of different metal cations, and were used to remove ammonium and manganese from aqueous solution. The results of the batch adsorption experiments indicated that akhtenskites effectively removed NH4+ and Mn2+, and the removal process fitted the pseudo-second-order model. By measuring the concentration of nitrate and nitrite, discriminating the adsorbed and oxidized Mn2+, and testing the zeta potential of the oxides, it can be concluded that NH4+ was merely removed by electrostatic adsorption via ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn–O−; Mn2+ could also be adsorbed by ion exchange with
Mn–O−; Mn2+ could also be adsorbed by ion exchange with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn–OH, and the adsorbed Mn2+ could be partly oxidized. The structural properties of the akhtenskites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) specific area, and X-ray photoelectron spectroscopy (XPS). The experimental results showed that ions with higher valence can result in a higher percentage of Mn(III) in akhtenskite. Mg2+ can result in a lower proportion of lattice oxygen in the oxide, and Fe3+ can increase the pH of the point of zero charge. Both of them were unfavored for the oxidation of Mn2+. Moreover, it was found that Ca–MnOx had optimal removal performance in the catalytic oxidation of Mn2+ owing to appropriate percentages of Olatt and Mn(III) and lower zeta potential. This study provides new insights into the synthesis and application of manganese oxides.
Mn–OH, and the adsorbed Mn2+ could be partly oxidized. The structural properties of the akhtenskites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) specific area, and X-ray photoelectron spectroscopy (XPS). The experimental results showed that ions with higher valence can result in a higher percentage of Mn(III) in akhtenskite. Mg2+ can result in a lower proportion of lattice oxygen in the oxide, and Fe3+ can increase the pH of the point of zero charge. Both of them were unfavored for the oxidation of Mn2+. Moreover, it was found that Ca–MnOx had optimal removal performance in the catalytic oxidation of Mn2+ owing to appropriate percentages of Olatt and Mn(III) and lower zeta potential. This study provides new insights into the synthesis and application of manganese oxides.


 Please wait while we load your content...
Please wait while we load your content...