Metal-free site-selective C–H cyanoalkylation of 8-aminoquinoline and aniline-derived amides with azobisisobutyronitrile†
Abstract
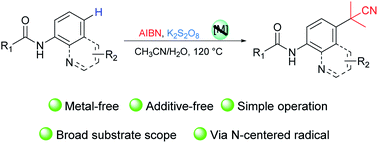
Using K2S2O8, an efficient and metal-free site-selective C–H cyanoalkylation of 8-aminoquinoline and aniline-derived amides with AIBN (azobisisobutyronitrile) was developed. Without any catalyst, various substrates and functional groups were compatible to afford corresponding products in moderate to high yields. A mechanism study displayed that a radical–radical coupling process was involved via the N-centered radical generation and delocalization of aryl amides.


 Please wait while we load your content...
Please wait while we load your content...