Development of rapid and selective epoxidation of α-pinene using single-step addition of H2O2 in an organic solvent-free process
Abstract
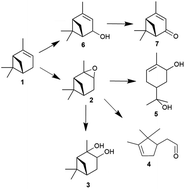
This study reports substantial improvement in the process for oxidising α-pinene, using environmentally friendly H2O2 at high atom economy (∼93%) and selectivity to α-pinene oxide (100%). The epoxidation of α-pinene with H2O2 was catalysed by tungsten-based polyoxometalates without any solvent. The variables in the screening parameters were temperatures (30–70 °C), oxidant amount (100–200 mol%), acid concentrations (0.02–0.09 M) and solvent types (i.e., 1,2-dichloroethane, toluene, p-cymene and acetonitrile). Screening the process parameters revealed that almost 100% selective epoxidation of α-pinene to α-pinene oxide was possible with negligible side product formation within a short reaction time (∼20 min), using process conditions of a 50 °C temperature in the absence of solvent and α-pinene/H2O2/catalyst molar ratio of 5 : 1 : 0.01. A kinetic investigation showed that the reaction was first-order for α-pinene and catalyst concentration, and a fractional order (∼0.5) for H2O2 concentration. The activation energy (Ea) for the epoxidation of α-pinene was ∼35 kJ mol−1. The advantages of the epoxidation reported here are that the reaction could be performed isothermally in an organic solvent-free environment to enhance the reaction rate, achieving nearly 100% selectivity to α-pinene oxide.


 Please wait while we load your content...
Please wait while we load your content...