Investigation on the interlayer coupling and bonding in layered nitride-halides ThNF and ThNCl
Abstract
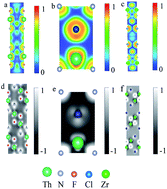
Motivated by recent experimental observation [N. Z. Wang, et al., Inorg. Chem., 2019, 58, 9897], we investigated the electronic properties and chemical bonding in layered nitride-halide compounds ThNF and ThNCl using first-principles calculations to illustrate the interlayer interaction. The energy gaps and chemical valences of both compounds are in agreement with experimental data. The crystal orbital Hamiltonian population (COHP) and charge density differential analysis show that interlayer chemical bonding plays a more important role than that van der Waals interactions in ThNF and ThNCl, in contrast to isostructural ZrNCl and HfNCl. These results explain why it is difficult to intercalate ThNF and ThNCl with charged particles, as observed in experiments.


 Please wait while we load your content...
Please wait while we load your content...