Self-supported Cu3P nanowire electrode as an efficient electrocatalyst for the oxygen evolution reaction†
Abstract
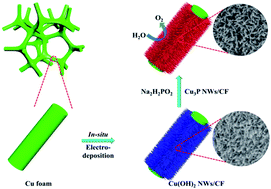
Hydrogen is an ideal energy carrier due to its abundant reserves and high energy density. Electrolyzing water is one of the carbon free technologies for hydrogen production, which is limited by the sluggish kinetics of the half reaction of the anode – the oxygen evolution reaction (OER). In this study, a self-supported Cu3P nanowire (Cu3P NWs/CF) electrode is prepared by electrodeposition of a Cu(OH)2 nanowire precursor on conductive Cu foam (Cu(OH)2 NWs/CF) with a subsequent phosphating procedure under a N2 atmosphere. When used as an OER working electrode in 1.0 M KOH solution at room temperature, Cu3P NWs/CF exhibits excellent catalytic performance with an overpotential of 327 mV that delivers a current density of 20 mA cm−2. Notably, it can run stably for 22 h at a current density of 20 mA cm−2 without obvious performance degradation. This highly efficient and stable OER catalytic performance is mainly attributed to the unique nanostructure and stable electrode construction. Interestingly, this synthesis strategy has been proved to be feasible to prepare large-area working electrodes (e.g. 40 cm−2) with unique nanowire structure. Therefore, this work has provided a good paradigm for the mass fabrication of self-supporting non-noble metal OER catalysts and effectively promoted the reaction kinetics of the anode of the electrolyzing water reaction.


 Please wait while we load your content...
Please wait while we load your content...