Solvent extraction of rare earths elements from nitrate media in DMDOHEMA/ionic liquid systems: performance and mechanism studies†
Abstract
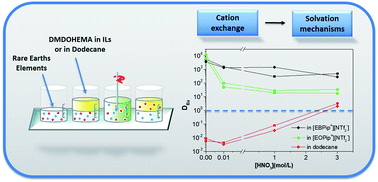
Extraction of La(III), Eu(III) and Fe(III) was compared in n-dodecane and two ionic liquids (ILs) (1-ethyl-1-butylpiperidinium bis (trifluoromethylsulfonyl)imide [EBPip+] [NTf2−] and 1-ethyl-1-octylpiperidinium bis (trifluoromethylsulfonyl)imide [EOPip+] [NTf2−]). Using the extractant N,N′-dimethyl-N,N′-dioctylhexylethoxymalonamide (DMDOHEMA), the effect of pH was investigated in detail to recover extraction mechanisms. The use of ILs as the organic solvent instead of n-dodecane, greatly enhances extraction efficiency, and an ionic liquid with a shorter alkyl chain [EBPip+] [NTf2−] provides higher extraction than [EOPip+] [NTf2−]. The mechanistic study points out that for low nitric acid concentrations ([HNO3] ≤ 0.01 M), metal is extracted via a cation of the ionic liquids, while for higher nitric acid concentrations ([HNO3] ≥ 1.0 M), extraction occurs through pure solvation mechanism of DMDOHEMA as in conventional diluents. This latter case is of high interest for applications, as higher extraction can be obtained without any loss of ILs by ion exchange mechanisms.
- This article is part of the themed collection: Renewable materials and recycling


 Please wait while we load your content...
Please wait while we load your content...