Ligand-free Pd/Ag-mediated dehydrogenative alkynylation of imidazole derivatives†
Abstract
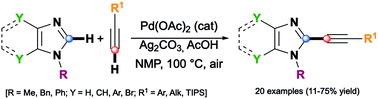
A variety of 2-alkynyl(benzo)imidazoles have been synthesized by dehydrogenative alkynylation of (benzo)imidazoles with terminal alkyne in NMP under air in the presence of Ag2CO3 as the oxidant and Pd(OAc)2 as the catalyst precursor. The data obtained in this study support a reaction mechanism involving a non-concerted metalation deprotonation (n-CMD) pathway.


 Please wait while we load your content...
Please wait while we load your content...