Synthesis and antitumor effects of novel benzyl naphthyl sulfoxide/sulfone derivatives derived from Rigosertib†
Abstract
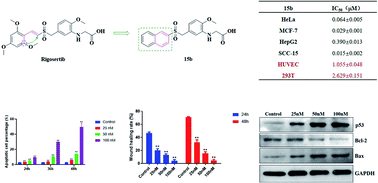
In this work, a series of novel benzyl naphthyl sulfoxides/sulfones derived from Rigosertib were designed and synthesized as potential antitumor agents. The in vitro cytotoxicity against four human cancer cell lines (HeLa, MCF-7, HepG2 and SCC-15) and two normal human cell lines (HUVEC and 293T) indicated that some of the sulfones and sulfoxides possessed potent antineoplastic activity that reached nanomolar levels and relatively low toxicity to normal cells. Among them, (2-methoxy-5-((naphthalen-2-ylsulfonyl)methyl)phenyl)glycine (15b) was found to be a promising antitumor drug candidate that could significantly inhibit tumor cell migration and induce tumor cell apoptosis via the p53-Bcl-2-Bax signaling pathway at nanomolar concentrations.


 Please wait while we load your content...
Please wait while we load your content...