Neuroinflammatory inhibitors from Gardneria nutans Siebold & Zuccarini†
Abstract
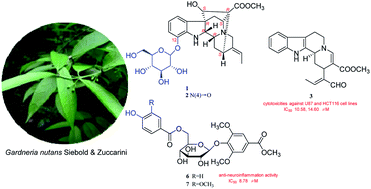
Two new monoterpene indole alkaloid glycosides nutanoside A–B (1–2), two new phenolic glycoside esters nutanester A–B (6–7), together with five known compounds (3–5, 8–9) were isolated from the ethanol extract of Gardneria nutans Siebold & Zuccarini. Their structures were established on the basis of extensive spectroscopic analysis and TDDFT/ECD calculations. Compounds 1 and 2 are two rare monoterpene indole alkaloids with the glucosyl moiety located at C-12 and represent the first two examples of enantiomer of ajmaline type monoterpene indole alkaloids. Compounds 3, 4 and 6 displayed significant inhibitory effects on NO production in over-activated BV2 microglial cells, with the IC50 values of 2.29, 6.36, and 8.78 μM, respectively. Compounds 1, 5, 7 could significantly inhibit the mRNA expression of inflammatory factors TNF-α and IL-6 induced by LPS in BV2 microglial cells at the effective concentration. Moreover, compound 3 exhibited stronger cytotoxicities against U87 and HCT116 cell lines than taxol with IC50 values of 10.58 and 14.60 μM, respectively.


 Please wait while we load your content...
Please wait while we load your content...