Solvent-free synthesis of symmetric methylene diesters via direct reaction of aromatic carboxylates with 1,n-dihaloalkanes†
Abstract
An efficient methodology for the synthesis of symmetrical methylene diesters was developed through direct reaction of various aromatic carboxylates with 1,n-dihaloalkanes under solvent-free conditions. This strategy offers a high product yield, facile work-up and purification, and an environmentally friendly approach to obtain long-chain methylene carboxylate scaffolds with increased diversity.
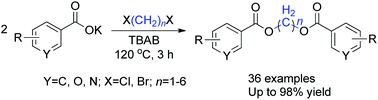


 Please wait while we load your content...
Please wait while we load your content...