Synthesis of covalent bonding MWCNT-oligoethylene linezolid conjugates and their antibacterial activity against bacterial strains†
Abstract
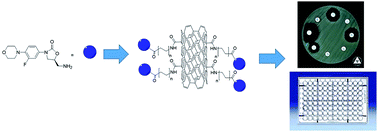
Nowadays, infectious diseases caused by drug-resistant bacteria have become especially important. Linezolid is an antibacterial drug active against clinically important Gram positive strains; however, resistance showed by these bacteria has been reported. Nanotechnology has improved a broad area of science, such as medicine, developing new drug delivery and transport systems. In this work, several covalently bounded conjugated nanomaterials were synthesized from multiwalled carbon nanotubes (MWCNTs), a different length oligoethylene chain (Sn), and two linezolid precursors (4 and 7), and they were evaluated in antibacterial assays. Interestingly, due to the intrinsic antibacterial activity of the amino-oligoethylene linezolid analogues, these conjugated nanomaterials showed significant antibacterial activity against various tested bacterial strains in a radial diffusion assay and microdilution method, including Gram negative strains as Escherichia coli (11 mm, 6.25 μg mL−1) and Salmonella typhi (14 mm, ≤0.78 μg mL−1), which are not inhibited by linezolid. The results show a significant effect of the oligoethylene chain length over the antibacterial activity. Molecular docking of amino-oligoethylene linezolid analogs shows a more favorable interaction of the S2-7 analog in the PTC of E. coli.


 Please wait while we load your content...
Please wait while we load your content...