Synthesis of optically active 2-substituted azetidine-2-carbonitriles from chiral 1-arylethylamine via α-alkylation of N-borane complexes†
Abstract
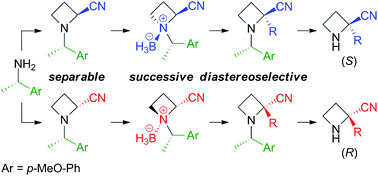
The base-promoted α-alkylation of N-((S)-1-arylethyl)azetidine-2-carbonitriles 3 via formation of their N-borane complexes 4 was investigated. For example, treatment of diastereomerically pure borane N-((S)-1′-(4′′-methoxyphenyl)ethyl)azetidine-2-carbonitrile complex (1S,2S,1′S)-4b with 1.2 equivalents of LDA at −78 °C followed by 1.3 equivalents of benzyl bromide at −78 °C and warming to room temperature produced α-benzylated (2S,1′S)-5ba in 72% yield and (2R,1′S)-5ba in 2% yield. A mechanism for this diastereoselective α-alkylation was proposed. Our method enables the production of optically active 2-substituted azetidine-2-carbonitriles, such as α-benzylated (S)-10a and (R)-10a, starting from commercially available (S)-(1-(4-methoxyphenyl)ethyl)amine.


 Please wait while we load your content...
Please wait while we load your content...