Fourier-transform infrared and X-ray diffraction analyses of the hydration reaction of pure magnesium oxide and chemically modified magnesium oxide†
Abstract
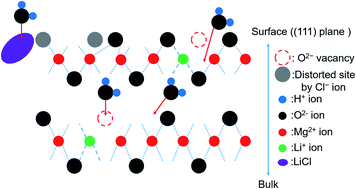
The magnesium hydroxide/magnesium oxide (Mg(OH)2/MgO) system is a promising chemical heat storage system that utilizes unused heat at the temperature range of 200–500 °C. We have previously reported that the addition of lithium chloride (LiCl) and/or lithium hydroxide (LiOH) promotes the dehydration of Mg(OH)2. The results revealed that LiOH primarily catalyzed the dehydration of the surface of Mg(OH)2, while LiCl promoted the dehydration of bulk Mg(OH)2. However, the roles of Li compounds in the hydration of MgO have not been discussed in detail. X-ray diffraction (XRD) and Fourier-transform infrared (FT-IR) techniques were used to analyze the effects of adding the Li compounds. The results revealed that the addition of LiOH promoted the diffusion of water into the MgO bulk phase and the addition of LiCl promoted the hydration of the MgO bulk phase. It was also observed that the concentration (number) of OH− affected hydration. The mechanism of hydration of pure and LiCl- (or LiOH)-added MgO has also been discussed.


 Please wait while we load your content...
Please wait while we load your content...