Methionine epimerization in cyclic peptides†
Abstract
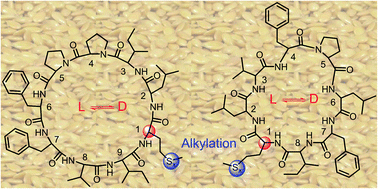
Bioactive flax cyclic octa- and nona-peptides containing single methionine (Met) and its oxidized forms were treated under mild alkaline conditions to perform regio-selective epimerization. Cyclic peptide epimerization at the Met α-proton in a single chemical step has not been reported previously. The epimerization rate varies among Met oxidation states and ring size. These D-amino isomers along with the developed Met alkylation strategy will enable an approach to novel chemical functionalization of biomolecules. The amino acid configurations were confirmed by Marfey derivatizations, and cytotoxicity studies show the difference among the isomers. These D-amino analogs can act as a potential biomarker in plant protein processing and biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...