ZnCl2-promoted domino reaction of 2-hydroxybenzonitriles with ketones for synthesis of 1,3-benzoxazin-4-ones†
Abstract
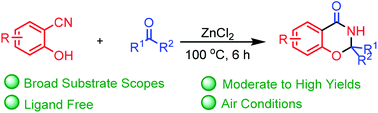
A ZnCl2-promoted synthesis of 1,3-benzoxazin-4-one from 2-hydroxybenzonitriles and ketones was developed. This method displays facile access to a diverse range of substituted 1,3-benzoxazin-4-ones in good yields. This synthetic protocol has advantages: (i) easy availability of starting material; (ii) strong corrosive acid-free condition; (iii) high yield.


 Please wait while we load your content...
Please wait while we load your content...