Structure-based design, synthesis, and biological evaluation of novel piperine–resveratrol hybrids as antiproliferative agents targeting SIRT-2†
Abstract
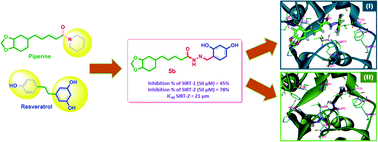
A series of novel piperine–resveratrol hybrids 5a–h was designed, synthesized, and structurally elucidated by IR, and 1H, 13C, and 19F NMR. Antiproliferative activities of 5a–h were evaluated by NCI against sixty cancer cell lines. Compound 5b, possessing resveratrol pharmacophoric phenolic moieties, showed a complete cell death against leukemia HL-60 (TB) and Breast cancer MDA-MB-468 with growth inhibition percentage of −0.49 and −2.83, respectively. In addition, 5b recorded significant activity against the other cancer cell lines with growth inhibition percentage between 80 to 95. New 5a–h hybrids were evaluated for their inhibitory activities against Sirt-1 and Sirt-2 as molecular targets for their antiproliferative action. Results showed that compounds 5a–h were more potent inhibitors of Sirt-2 than Sirt-1 at 5 μm and 50 μm. Compound 5b showed the strongest inhibition of Sirt-2 (78 ± 3% and 26 ± 3% inhibition at 50 μM and 5 μM, respectively). Investigation of intermolecular interaction via Hirschfeld surface analysis indicates that these close contacts are mainly ascribed to the O–H⋯O hydrogen bonding. To get insights into the Sirt-2 inhibitory mechanism, a docking study was performed where 5b was found to fit nicely inside both extended C-pocket and selectivity pocket and could compete with the substrate acyl-Lys. Another possible binding pattern showed that 5b could act by partial occlusion of the NAD+ C-pocket. Collectively, these findings would contribute significantly to better understanding the Sirt-2 inhibitory mechanism in order to develop a new generation of refined and selective Sirt-2 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...