Color-tunable arylaminoanthraquinone dyes through hydrogen-bond-assisted charge transfer interaction†
Abstract
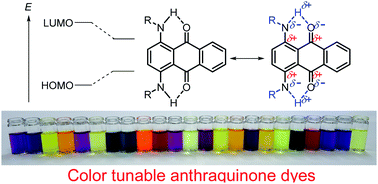
We prepared a series of arylaminoanthraquinone derivatives, including those with electron-accepting sulfone units and/or with electron-donating dialkylamino units. A color-tunable anthraquinone library that reached into the NIR region could be prepared through the precise control of frontier orbitals. Fine color-tuning was achieved through proper selection and positioning of the substituents. Effective intramolecular hydrogen-bond-assisted charge transfer interaction between electron-donating aniline/p-phenylenediamine and electron-accepting anthraquinone substructures induced a significant bathochromic shift of anthraquinone. The number and position of the substituents and the molecular conformation also significantly contributed to determining photophysical properties.


 Please wait while we load your content...
Please wait while we load your content...