Computational insights into Ir(iii)-catalyzed allylic C–H amination of terminal alkenes: mechanism, regioselectivity, and catalytic activity†
Abstract
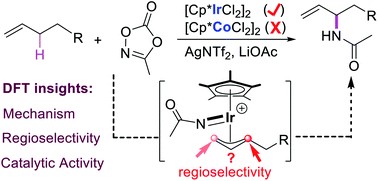
Computational studies on Ir(III)-catalyzed intermolecular branch-selective allylic C–H amination of terminal olefins with methyl dioxazolone have been carried out to investigate the mechanism, including the origins of regioselectivity and catalytic activity difference. The result suggests that the reaction proceeds through generation of active species, alkene coordination, allylic C–H activation, decarboxylation, migratory insertion, and protodemetalation. The presence of AgNTf2 could thermodynamically promote the formation of catalytically active species [Cp*Ir(OAc)]+. Both the weaker Ir–C(internal) bond and the closer interatomic distance of N⋯C(internal) in the key allyl-Ir(V)-nitrenoid intermediate make the migratory insertion into Ir–C(internal) bond easier than into the Ir–C(terminal) bond, leading to branch-selective allylic C–H amidation. The high energy barrier for allylic C–H activation in the Co system could account for the observed sluggishness, which is mainly ascribed to the weaker coordination capacity of alkenes to the triplet Cp*Co(OAc)+ and the deficient metal⋯H interaction to assist hydrogen transfer.


 Please wait while we load your content...
Please wait while we load your content...