Catalysis of electro-oxidation of antibiotics by nitroxyl radicals and the electrochemical sensing of vancomycin†
Abstract
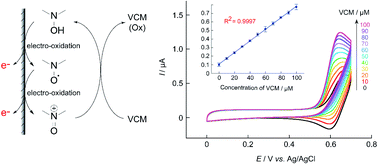
Quantifying drug concentrations in vivo quickly and easily is possible using electrochemical methods. The present study describes the electrochemical detection of vancomycin (VCM) and other antibiotics from the current obtained using nitroxyl radicals as electrocatalysts. Nortropine N-oxyl (NNO), which is more active than 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO), a typical nitroxyl radical compound, produced greater current values for drugs with intramolecular hydroxy groups and secondary and tertiary amines. However, because the catalytic action of NNO is inactivated by primary amines in the substrate, VCM and teicoplanin with primary amines could not be detected. TEMPO was less active than NNO but not inactivated against primary amines. Therefore, electrochemical sensing of vancomycin was done using 4-acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl (A-TEMPO), which has a greater oxidation capacity than TEMPO due to its electron-withdrawing groups. As a result, the current of A-TEMPO increased in the low concentration range of VCM as compared to TEMPO. This method also was able to quantify VCM in the concentration range of 10–100 μM, which is an important concentration range for drug monitoring in blood.


 Please wait while we load your content...
Please wait while we load your content...