Selenium and sulphur reactions involved in manganese reduction from sulphate solutions
Abstract
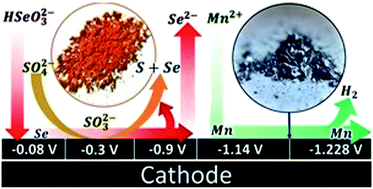
Electrochemical reduction of ionic species during manganese deposition from sulphated aqueous solutions has been studied in an electrochemical reactor with two anionic exchange membranes. Thermodynamic analysis, voltammetries, and chronopotentiometries were used to determine the reaction mechanism of the reductions developed, with the results demonstrating that the effect of the elemental selenium on the hydrogen evolution leads to the formation of elemental sulphur by reducing the sulphate ions with both membranes. It was also evident that in the range of −25 to −50 A m−2 the electrodeposition of metallic manganese begins, with minimal interference from parasitic reactions.


 Please wait while we load your content...
Please wait while we load your content...