Protein detection enabled using functionalised silk-binding peptides on a silk-coated optical fibre†
Abstract
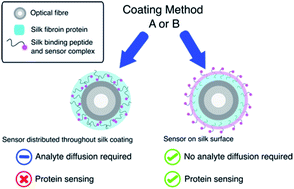
We present a new coating procedure to prepare optical fibre sensors suitable for use with protein analytes. We demonstrate this through the detection of AlexaFluor-532 tagged streptavidin by its binding to D-biotin that is functionalised onto an optical fibre, via incorporation in a silk fibroin fibre coating. The D-biotin was covalently attached to a silk-binding peptide to provide SBP–biotin, which adheres the D-biotin to the silk-coated fibre tip. These optical fibre probes were prepared by two methods. The first involves dip-coating the fibre tip into a mixture of silk fibroin and SBP–biotin, which distributes the SBP–biotin throughout the silk coating (method A). The second method uses two steps, where the fibre is first dip-coated in silk only, then SBP–biotin added in a second dip-coating step. This isolates SBP–biotin to the outer surface of the silk layer (method B). A series of fluorescence measurements revealed that only the surface bound SBP–biotin detects streptavidin with a detection limit of 15 μg mL−1. The fibre coatings are stable to repeated washing and long-term exposure to water. Formation of silk coatings on fibres using commercial aqueous silk fibroin was found to be inhibited by a lithium concentration of 200 ppm, as determined by atomic absorption spectroscopy. This was reduced to less than 20 ppm by dialysis against water, and was found to successfully form a coating on optical fibres.


 Please wait while we load your content...
Please wait while we load your content...