Chemical structure stabilities of a SixFy (x ≤ 6, y ≤ 12) series†
Abstract
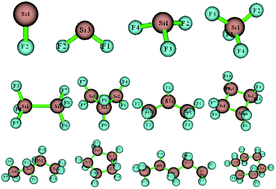
In this paper, we construct a SixFy (x ≤ 6, y ≤ 12) series optimised at the B3LYP/6-31G(d,p) level. At the same level, we perform frontline molecular orbital (FMO), Mayer bond order (MBO), molecular surface electrostatic potential (MS-EPS) and natural population analysis (NPA) calculations to study the chemical structure stabilities of these SixFy molecules. The FMO and MBO results demonstrate that the chemical structure stabilities of the SixFy (x ≤ 6, y ≤ 12) series are ranked (from strong to weak) as SiF4 > Si2F6 > Si3F8 > Si4F10 > SiF2 > Si5F12 > Si3F6 (ring) > Si5F10 (ring) > Si6F12 (ring) > Si4F8 (ring). Furthermore, the chemical structure stabilities of the chains are stronger than those of the rings, while the number of silicon atoms is the same. In addition, infrared spectroscopy analysis shows that SiF4 is the most stable among the SixFy (x ≤ 6, y ≤ 12) series, followed by Si2F6, and SiF2 is unstable. The experimental results are consistent with theoretical calculations. Finally, the MS-EPS and NPA results indicate that compounds in the SixFy (x ≤ 6, y ≤ 12) series tend to be attacked by nucleophiles rather than by electrophiles; also, they show poor chemical structure stability when encountering nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...