Binding modes of methyl α-d-glucopyranoside to an artificial receptor in crystalline complexes†
Abstract
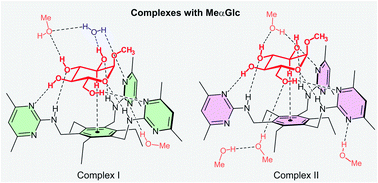
Compared to the numerous X-ray crystal structures of protein-carbohydrate complexes, the successful elucidation of the crystal structures of complexes between artificial receptors and carbohydrates has been very rarely reported in the literature. In this work, we describe the binding modes of two complexes formed between methyl α-D-glucopyranoside and an artificial receptor belonging to the class of compounds consisting of a 1,3,5-trisubstituted 2,4,6-trialkylbenzene scaffold. It is particularly noteworthy that these two complexes are present in one crystal structure, as was observed by us for the first time in the case of the recently reported three crystal structures of the complexes with methyl β-D-glucopyranoside, each containing two different receptor–carbohydrate complexes. The noncovalent interactions stabilizing the new complexes are compared with those observed in the aforementioned crystalline complexes with methyl β-D-glucopyranoside.


 Please wait while we load your content...
Please wait while we load your content...