μ-Oxo-bridged diiron(iii) complexes of tripodal 4N ligands as catalysts for alkane hydroxylation reaction using m-CPBA as an oxidant: substrate vs. self hydroxylation†
Abstract
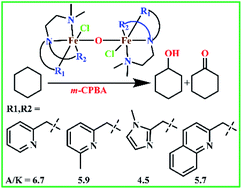
A series of non-heme μ-oxo-bridged dinuclear iron(III) complexes of the type [Fe2(μ-O)(L1–L6)2Cl2]Cl2 1–6 have been isolated and their catalytic activity towards oxidative transformation of alkanes into alcohols has been studied using m-choloroperbenzoic acid (m-CPBA) as an oxidant. All the complexes were characterized by CHN, electrochemical, and UV-visible spectroscopic techniques. The molecular structures of 2 and 5 have been determined successfully by single crystal X-ray diffraction analysis and both possesses octahedral coordination geometry and each iron atom is coordinated by four nitrogen atoms of the 4N ligand and a bridging oxygen. The sixth position of each octahedron is coordinated by a chloride ion. The (μ-oxo)diiron(III) core is linear in 2 (Fe–O–Fe, 180.0°), whereas it is non-linear (Fe–O–Fe, 161°) in 5. All the diiron(III) complexes show quasi-reversible one electron transfer in the cyclic voltammagram and catalyze the hydroxylation of alkanes like cyclohexane, adamantane with m-CPBA as an oxidant. In acetonitrile solution, adding excess m-CPBA to the diiron(III) complex 2 without chloride ions leads to intramolecular hydroxylation reaction of the oxidant. Interestingly, 2 catalyzes alkane hydroxylation in the presence of chloride ions, but intramolecular hydroxylation in the absence of chloride ions. The observed selectivity for cyclohexane (A/K, 5–7) and adamantane (3°/2°, 9–18) suggests the involvement of high-valent iron–oxo species rather than freely diffusing radicals in the catalytic reaction. Moreover, 4 oxidizes (A/K, 7) cyclohexane very efficiently up to 513 TON while 5 oxidizes adamantane with good selectivity (3°/2°, 18) using m-CPBA as an oxidant. The electronic effects of ligand donors dictate the efficiency and selectivity of catalytic hydroxylation of alkanes.


 Please wait while we load your content...
Please wait while we load your content...