In situ gelation of aqueous sulfuric acid solution for fuel cells
Abstract
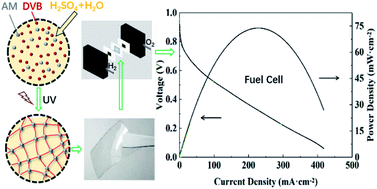
Aqueous sulfuric acid solution is a versatile liquid electrolyte for electrochemical applications and gelation of it has the advantages of easy shaping and reduced leaking. Herein, aqueous sulfuric acid solutions with concentrations of 1–4 mol L−1 are fabricated into gel membranes by in situ polymerization of acrylamide as a monomer and divilynbenzene as a crosslinker for fuel cell applications. The gel membrane with an acid concentration of 3.5 mol L−1 exhibited the maximum proton conductivity of 184 mS cm−1 at 30 °C. Tensile fracture strength of the gel membrane reached 53 kPa with a tensile strain of 14. Thermogravimetric analysis reveals that the gel membranes are thermally stable at temperatures up to 231 °C. The gel membranes are successfully assembled into fuel cells and a peak power density of 74 mW cm−2 is achieved. The fuel cell maintains steady operation over 200 h. In situ gelation of aqueous sulfuric acid solution offers an efficient strategy to prepare gel electrolytes for electrochemical devices.


 Please wait while we load your content...
Please wait while we load your content...