Competing HB acceptors: an extensive NMR investigations corroborated by single crystal XRD and DFT calculations†
Abstract
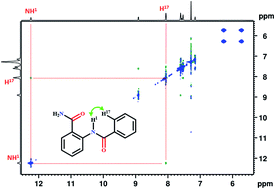
A series of N-benzoylanthranilamide derivatives have been synthesized with the substitution of competitive HB acceptors and investigated by NMR spectroscopy and single crystal XRD. The interesting rivalry for HB acceptance between ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and X (F or OMe) is observed in the investigated molecules which leads to an unusual increase in the electron density at the site of one of the NH protons, reflecting in the high field resonance in the 1H NMR spectrum. The NMR experimental findings and single crystal XRD are further reinforced by the DFT studies.
O and X (F or OMe) is observed in the investigated molecules which leads to an unusual increase in the electron density at the site of one of the NH protons, reflecting in the high field resonance in the 1H NMR spectrum. The NMR experimental findings and single crystal XRD are further reinforced by the DFT studies.


 Please wait while we load your content...
Please wait while we load your content...