Magnetic GO/Fe3O4 for rapid malachite green (MG) removal from aqueous solutions: a reversible adsorption†
Abstract
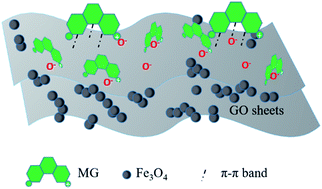
Magnetic GO/Fe3O4 was synthesized using co-precipitation of Fe2+ and Fe3+ composited with graphene oxide (GO) in alkaline conditions. SEM, XPS, FTIR, N2 adsorption and VSM techniques were employed to characterize the surface peculiarities of GO/Fe3O4 and it was then used for removal of malachite green (MG). The key influencing factors on adsorption, such as mass ratio of GO, pH value and dosage of GO/Fe3O4, were investigated. The Freundlich isotherm was well fitted to the experimental data, suggesting GO/Fe3O4 has more than one type of reactive site. By comparing the adsorption of anionic dyes and cationic dyes onto GO/Fe3O4, it was concluded that GO/Fe3O4 could be extensively applied to take up cationic dyes mainly for electrostatic interaction. In addition, the spent GO/Fe3O4 was almost 100% recovered in a water bath at 80 °C. An ultraviolet-visible (UV-vis) spectrophotometer and an atom adsorption spectrophotometer (AAS) were used to determine leached GO and Fe ions discharged into the treated solutions. Low leaching showed that magnetic GO/Fe3O4 is a stable environmentally-friendly material.


 Please wait while we load your content...
Please wait while we load your content...