Access to 3,3-disubstituted oxindoles via microwave-assisted Cannizzaro and aldol reactions of formaldehyde with isatins and their imines†
Abstract
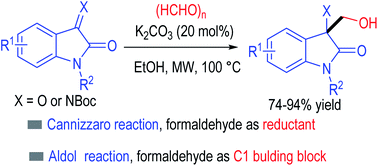
3,3-Disubstituted oxindoles are important structure motifs in natural products and pharmaceutical agents. Here we disclose a simple and direct access to this class of molecules by using readily available formaldehyde and isatins (and their imines) as the substrates. The reaction proceeds with the assistance of microwave heating in the presence of a mild base. Formaldehyde behaves as both a reductant (via a Cannizzaro process with isatin) and an electrophile.


 Please wait while we load your content...
Please wait while we load your content...