Palladium-catalyzed one-pot synthesis of 2-substituted quinazolin-4(3H)-ones from o-nitrobenzamide and alcohols†
Abstract
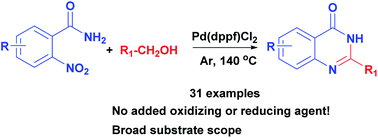
Palladium-catalyzed 2-substituted quinazolin-4(3H)-one formation from readily available o-nitrobenzamides and alcohols using hydrogen transfer is described. Various quinazolin-4(3H)-ones were obtained in good to high yields. The cascade reaction including alcohol oxidation, nitro reduction, condensation, and dehydrogenation occurs without any added reducing or oxidizing agent.


 Please wait while we load your content...
Please wait while we load your content...