Exploring synthetic and therapeutic prospects of new thiazoline derivatives as aldose reductase (ALR2) inhibitors†
Abstract
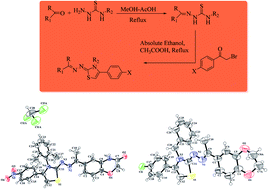
Inhibition of aldose reductase (ALR2) by using small heterocyclic compounds provides a viable approach for the development of new antidiabetic agents. With our ongoing interest towards aldose reductase (ALR2) inhibition, we have synthesized and screened a series of thiazoline derivatives (5a–k, 6a–f, 7a–1 & 8a–j) to find a lead as a potential new antidiabetic agent. The bioactivity results showed the thiazoline-based compound 7b having a benzyl substituent and nitrophenyl substituent-bearing compound 8e were identified as the most potent molecules with IC50 values of 1.39 ± 2.21 μM and 1.52 ± 0.78 μM respectively compared with the reference sorbinil with an IC50 value of 3.14 ± 0.02 μM. Compound 7b with only 23.4% inhibition for ALR1 showed excellent selectivity for the targeted ALR2 to act as a potential lead for the development of new therapeutic agents for diabetic complications.


 Please wait while we load your content...
Please wait while we load your content...