Elucidating the mechanism and origins of selectivity on catalyst-dependent cyclization reactions to form polycyclic indolines from a theoretical study†
Abstract
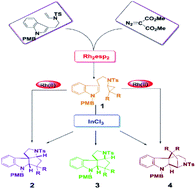
There is a significant role for bioactive polycyclic indolines in the pharmaceutical science field. In this paper, a systematic DFT study at the M06-D3/SMD/BS2//B3LYP-D3/BS1 level is adopted to investigate the cyclization reaction catalyzed by Rh2(esp)2 and InCl3 to generate polycyclic indolines. Luckily, the simplification of the Rh2(esp)2 computational model is feasible, and successfully used in this study. The computational results detailed indicate the reaction mechanisms catalyzed by different catalysts, and the regio- and diastereo-selectivity. The regio-selectivity is controlled by the weak interaction (reflected in repulsive interaction) of the key transition state in the InCl3-catalyzed pathway, and the larger distortion energy makes the regio-selectivity more obvious in the pathway catalyzed by Rh2(esp)2. It is important that this theoretical study suggests the significance of the catalyst in the reaction system in detail by NBO and FMO analysis. This paper is a good explanation of the experimental phenomenon caused by the catalyst where InCl3 is more significant than Rh2(esp)2. The reaction mechanism and the importance of the catalysts are revealed in detail by this particular theoretical study.


 Please wait while we load your content...
Please wait while we load your content...