A hydrothermal synthesis of Ru-doped LiMn1.5Ni0.5O4 cathode materials for enhanced electrochemical performance
Abstract
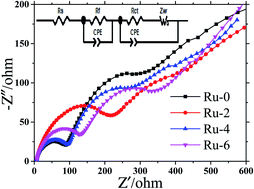
An Ru-doped spinel-structured LiNi0.5Mn1.5O4 (LNMO) cathode has been prepared via a simple hydrothermal synthesis method. The as-prepared cathode is characterized via Fourier transform infrared (FTIR) spectroscopy, powder X-ray diffraction (XRD), scanning electron microscopy (SEM), laser particle size distribution analysis, X-ray photoelectron spectroscopy (XPS) and electrochemistry performance tests. The FTIR spectroscopy and XRD analyses show that the Ru-doped LNMO has a good crystallinity with a disordered Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group structure. The disordered structure in the cathode increased and the LixNi1−xO impurity phase decreased when Ru addition increased. SEM shows that all samples are octahedral particles with homogeneous sizes distribution, and the particle size analysis shows that the Ru-doped samples have smaller particle size. XPS confirms the existence of Ru ions in the sample, and reveals that the Ru induce to part of Mn4+ transfers to Mn3+ in the LNMO. The electrochemical property indicated that the Ru-doped cathode exhibits better electrochemical properties in terms of discharge capacity, cycle stability and rate performance. At a current density of 50 mA g−1, the discharge specific capacity of the Ru-4 sample is 140 mA h g−1, which is much higher than that of the other samples. It can be seen from the rate capacity curves that the Ru-doped samples exhibit high discharge specific capacity, particularly at high current density.
m space group structure. The disordered structure in the cathode increased and the LixNi1−xO impurity phase decreased when Ru addition increased. SEM shows that all samples are octahedral particles with homogeneous sizes distribution, and the particle size analysis shows that the Ru-doped samples have smaller particle size. XPS confirms the existence of Ru ions in the sample, and reveals that the Ru induce to part of Mn4+ transfers to Mn3+ in the LNMO. The electrochemical property indicated that the Ru-doped cathode exhibits better electrochemical properties in terms of discharge capacity, cycle stability and rate performance. At a current density of 50 mA g−1, the discharge specific capacity of the Ru-4 sample is 140 mA h g−1, which is much higher than that of the other samples. It can be seen from the rate capacity curves that the Ru-doped samples exhibit high discharge specific capacity, particularly at high current density.


 Please wait while we load your content...
Please wait while we load your content...