Enhanced simultaneous adsorption of As(iii), Cd(ii), Pb(ii) and Cr(vi) ions from aqueous solution using cassava root husk-derived biochar loaded with ZnO nanoparticles
Abstract
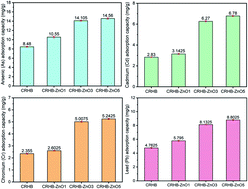
This study presents the modification of cassava root husk-derived biochar (CRHB) with ZnO nanoparticles (ZnO-NPs) for the simultaneous adsorption of As(III), Cd(II), Pb(II) and Cr(VI). By conducting batch-mode experiments, it was concluded that 3% w/w was the best impregnation ratio for the modification of CRHB using ZnO-NPs, and was denoted as CRHB-ZnO3 in this study. The optimal conditions for heavy metal adsorption were obtained at a pH of 6–7, contact time of 60 min, and initial metal concentration of 80 mg L−1. The heavy metal adsorption capacities onto CRHB-ZnO3 showed the following tendency: Pb(II) > Cd(II) > As(III) > Cr(VI). The total optimal adsorption capacity achieved in the adsorption of the 4 abovementioned metals reached 115.11 and 154.21 mg g−1 for CRHB and CRHB-ZnO3, respectively. For each Pb(II), Cd(II), As(III), and Cr(VI) metal, the maximum adsorption capacities of CRHB-ZnO3 were 44.27, 42.05, 39.52, and 28.37 mg g−1, respectively, and those of CRHB were 34.47, 32.33, 26.42 and 21.89 mg g−1, respectively. In terms of kinetics, both the pseudo-first-order and the pseudo-second-order fit well with metal adsorption onto biochars with a high correlation coefficient of R2, while the best isothermal description followed the Langmuir model. As a result, the adsorption process of heavy metals onto biochars was chemisorption on homogeneous monolayers, which was mainly controlled by cation exchange and surface precipitation mechanisms due to enriched oxygen-containing surface groups with ZnO-NP modification of biochar. The FTIR and EDS analysis data confirmed the important role of oxygen-containing surface groups, which significantly contributed to removal of heavy metals with extremely high adsorption capacities, comparable with other studies. In conclusion, due to very high adsorption capacities for metal cations, the cassava root husk-derived biochar modified with ZnO-NPs can be applied as the alternative, inexpensive, non-toxic and highly effective adsorbent in the removal of various toxic cations.


 Please wait while we load your content...
Please wait while we load your content...