Retracted Article: Experimental and surface morphological studies of corrosion inhibition on carbon steel in HCl solution using some new hydrazide derivatives
Abstract
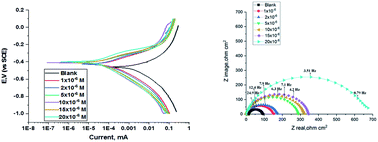
The corrosion inhibition of C-steel in 1 M HCl was assessed using three newly synthesized hydrazide derivatives (H1, H2 and H3) using weight loss (WL), potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) techniques. Also, the adsorption of these compounds was confirmed using several techniques such as atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). High inhibition efficiencies were obtained resulting from the constitution of the protective layer on the C-steel surface, which increased with increasing concentration and temperature and reached 91.7 to 96.5% as obtained from the chemical method at 20 × 10−6 M at 45 °C. The polarization curves refer to these derivatives belonging to mixed-type inhibitors. The adsorption of (H1, H2 and H3)on the CS surface follows the Temkin adsorption isotherm. Inhibition influence of hydrazide derivatives at the molecular level was greatly proven using quantum chemical calculations and Monte Carlo simulation methods. Furthermore, the molecular simulation results evidenced the adsorption of these derivatives on the carbon steel surface.


 Please wait while we load your content...
Please wait while we load your content...