Is natural fraxin an overlooked radical scavenger?†
Abstract
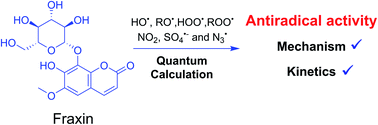
Fraxin (FX) (7-hydroxy-6-methoxycoumarin 8-glucoside) is a typical natural product of the coumarin family. This compound was shown to protect endothelial cells from oxidative stress; however, the nature of its antioxidant properties is still ambiguous. In this study, we report on a systematic evaluation of the radical scavenging activity of FX using a two-tier protocol based on thermodynamic and kinetic calculations. The results show that FX has moderate activity in the aqueous physiological environment against a range of radicals including HO˙, CCl3O˙, CCl3OO˙, NO2, 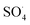 ,
, 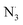 and HOO˙. The latter was examined in detail due to the prevalence of HOO˙ as a source of oxidative stress in biological systems. HOO˙ scavenging activity was promising in the gas phase but low in physiological environments with koverall = 1.57 × 106, 3.13 × 102 and 2.68 × 103 M−1 s−1 in the gas phase, pentyl ethanoate and water solvents, respectively. The formal hydrogen transfer mechanism at the O7–H bond dominates the hydroperoxyl radical scavenging of FX in the nonpolar media, whereas, in the polar environment, the activity is exerted by the single electron transfer mechanism of the anion state. This activity falls behind typical antioxidants such as Trolox, ascorbic acid, and trans-resveratrol under the studied conditions. Thus FX may have multiple health benefits, but it is not an outstanding natural antioxidant.
and HOO˙. The latter was examined in detail due to the prevalence of HOO˙ as a source of oxidative stress in biological systems. HOO˙ scavenging activity was promising in the gas phase but low in physiological environments with koverall = 1.57 × 106, 3.13 × 102 and 2.68 × 103 M−1 s−1 in the gas phase, pentyl ethanoate and water solvents, respectively. The formal hydrogen transfer mechanism at the O7–H bond dominates the hydroperoxyl radical scavenging of FX in the nonpolar media, whereas, in the polar environment, the activity is exerted by the single electron transfer mechanism of the anion state. This activity falls behind typical antioxidants such as Trolox, ascorbic acid, and trans-resveratrol under the studied conditions. Thus FX may have multiple health benefits, but it is not an outstanding natural antioxidant.


 Please wait while we load your content...
Please wait while we load your content...