Practical scale up synthesis of carboxylic acids and their bioisosteres 5-substituted-1H-tetrazoles catalyzed by a graphene oxide-based solid acid carbocatalyst†
Abstract
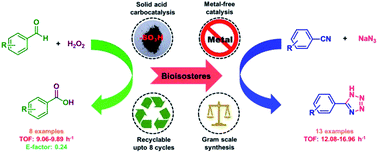
Herein, catalytic application of a metal-free sulfonic acid functionalized reduced graphene oxide (SA-rGO) material is reported for the synthesis of both carboxylic acids and their bioisosteres, 5-substituted-1H-tetrazoles. SA-rGO as a catalytic material incorporates the intriguing properties of graphene oxide material with additional benefits of highly acidic sites due to sulfonic acid groups. The oxidation of aldehydes to carboxylic acids could be efficiently achieved using H2O2 as a green oxidant with high TOF values (9.06–9.89 h−1). The 5-substituted-1H-tetrazoles could also be effectively synthesized with high TOF values (12.08–16.96 h−1). The synthesis of 5-substituted-1H-tetrazoles was corroborated by single crystal X-ray analysis and computational calculations of the proposed reaction mechanism which correlated well with experimental findings. Both of the reactions could be performed efficiently at gram scale (10 g) using the SA-rGO catalyst. SA-rGO displays eminent reusability up to eight runs without significant decrease in its productivity. Thus, these features make SA-rGO riveting from an industrial perspective.


 Please wait while we load your content...
Please wait while we load your content...