Unexpected ortho C–H bond activation in coordinated 7,8-benzoquinoline: synthesis and characterisation of heteroleptic Ir(iii)-7,8-benzoquinoline complexes†
Abstract
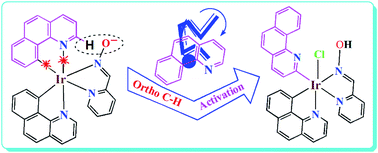
Two iridium(III) complexes were isolated via the reaction of pyridine-2-aldoxime (Hpyrald) with 7,8-benzoquinoline (benzq)-derived iridium starting material, namely [(benzq)2Ir(μ-Cl)2Ir(benzq)2] (1). Among the two complexes, [IrIII(benzq)2(pyrald)] (2) and [IrIII(benzq-κN,κC10)(benzq-κC2)(Hpyrald)(Cl)] (3), the later displayed unusual ortho C–H bond activation in one of the coordinated 7,8-benzoquinoline rings. The complex (2) presented a usual structure as expected.


 Please wait while we load your content...
Please wait while we load your content...