Synthesis of novel seven-membered carbasugars and evaluation of their glycosidase inhibition potentials†
Abstract
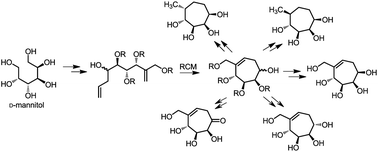
Here, we report the synthesis of five novel seven-membered carbasugar analogs. We adopted a chiral-pool strategy starting from the cheap and readily available D-mannitol to synthesize these ring-expanded carbasugars. Apart from several regioselective protecting group manipulations, these syntheses involved Wittig olefination and ring-closing metathesis as the key steps. We observed an unprecedented deoxygenation reaction of an allylic benzyl ether upon treatment with H2/Pd during the synthesis. Preliminary biological evaluation of the carbasugars revealed that these ring expanded carbasugars act as inhibitors of various glycosidases. This study highlights the importance of the synthesis of novel ring expanded carbasugars and their biological exploration.


 Please wait while we load your content...
Please wait while we load your content...