Mesoporous adsorbent for competitive adsorption of fluoride ions in zinc sulfate solution
Abstract
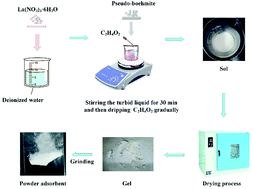
Al–La hybrid gel was constructed using an innovative acid-catalyzed and calcination free sol–gel formation process which only included a sol–gel process lasting for 30 min and a drying procedure at 150 °C. This novel material was used as an adsorbent for competitive adsorption of fluoride ions in zinc sulfate solution. The properties, optimal adsorption conditions, synthetic principle and adsorption mechanism of the material were systemically investigated. The results showed that γ-AlO(OH) composed the skeleton of the Al–La hybrid gel and La(CH3COO)3 was embedded in the framework, which formed large amounts of ink-bottle type mesopores. A high fluoride ion adsorption rate with the removal rate reaching 50.88% within 1 min at 50 °C, 3 g L−1 was obtained. Analysis of the adsorption data has demonstrated that the adsorption of fluoride ions by the Al–La hybrid gel followed pseudo-second-order kinetics. Moreover, both Langmuir and Freundlich models can describe the adsorption process well. The maximum adsorption capacity of the Al–La hybrid adsorbent was 28.383 mg g−1. Furthermore, the mechanism analysis results indicated that the fluoride ions were mainly removed by the electrostatic adsorption on the AlO(OH), and a small amount of fluoride ions was also adsorbed by the complexation of lanthanum and fluoride ions. Since both AlO(OH) and La(CH3COO)3 had a large number of fluoride ion adsorption sites, the Al–La hybrid gel obtained an ideal adsorption capacity. In addition, the adsorption rate was greatly enhanced by the capillary action existing from the initial to the final stage of adsorption.


 Please wait while we load your content...
Please wait while we load your content...