Synthesis and biological evaluation of novel artemisone–piperazine–tetronamide hybrids†
Abstract
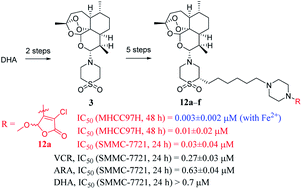
For the first time, six novel artemisone–piperazine–tetronamide hybrids (12a–f) were efficiently synthesised from dihydroartemisinin (DHA) and investigated for their in vitro cytotoxicity against some human cancer cells and benign cells. All the targets showed good cytotoxic activity in vitro. Hybrid 12a exhibited much better inhibitory activity against human liver cancer cell line SMMC-7721 (IC50 = 0.03 ± 0.04 μM for 24 h) than the parent DHA (IC50 > 0.7 μM), and two references, vincristine (VCR; IC50 = 0.27 ± 0.03 μM) & cytosine arabinoside (ARA; IC50 = 0.63 ± 0.04 μM). Furthermore, hybrid 12a had low toxicity against human benign liver cell line LO2 (IC50 = 0.70 ± 0.02 μM for 24 h) compared with VCR, ARA, and DHA in vitro. Moreover, the inhibitory activity of hybrid 12a was obviously enhanced when human liver cancer cell line MHCC97H absorbed Fe2+ in vitro.


 Please wait while we load your content...
Please wait while we load your content...