Mechanistic insights into the C–H activation of methane mediated by the unsupported and silica-supported VO2OH and CrOOH: a DFT study†
Abstract
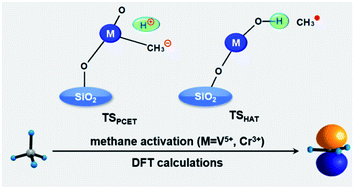
The direct activation and conversion of methane has been a topic of interest in both academia and industry for several decades. Deep understanding of the corresponding mechanism and reactivity mediated by diverse catalytic clusters, as well as the supporting materials, is still highly desired. In this work, the regulation mechanism of C–H bond activation of methane, mediated by the closed-shell VO2OH, the open-shell CrOOH, and their silica supported clusters, has been investigated by density functional theory (DFT) calculations. The hydrogen-atom transfer (HAT) reaction towards methane C–H bond activation is more feasible when mediated by the unsupported/silica-supported CrOOH clusters versus the VO2OH clusters, due to the intrinsic spin density located on the terminal Ot atom. The proton-coupled electron transfer (PCET) pathways are regulated by both the nucleophilicity of the Ot site and the electrophilicity of the metal center, which show no obvious difference in energy consumption among the four reactions examined. Moreover, the introduction of a silica support can lead to subtle influences on the intermolecular interaction between the CH4 molecule and the catalyst cluster, as well as the thermodynamics of the methane C–H activation.


 Please wait while we load your content...
Please wait while we load your content...