Reactions of aryl dimethylphosphinothioate esters with anionic oxygen nucleophiles: transition state structure in 70% water–30% ethanol†
Abstract
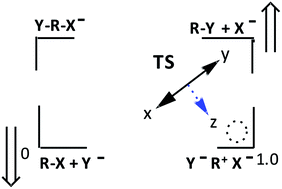
Aryl dimethylphosphinates, 2, react with anionic oxygen nucleophiles in water via a concerted (ANDN) mechanism. With EtO− in anhydrous ethanol, the mechanism is associative (AN + DN), with rate-limiting pentacoordinate intermediate formation. This change in mechanism with solvent change has been ascribed to changes in the nucleophile and leaving group basicities accompanying solvent change. This paper reports on a kinetic analysis of the reactions of the aryl dimethylphosphinothioates, 3a–g, with oxygen nucleophiles in 70% water–30% ethanol (v/v) solvent at 25 °C, reactions known to proceed by a concerted mechanism in water, to test the rationalization stated above, since the nucleophiles and LGs of interest are more basic in aqueous ethanol than in water. The change in solvent causes an ca. 14 to 320-fold decrease in rate. Hammett and Brønsted-type correlations characterize a concerted TS with less P–LG bonding in aqueous ethanol than in water. Two opposing consequences are associated with the solvent change: (a) increased basicity of nucleophiles and LGs, which lead to a modest tightening of the TS; and (b) better stabilization of the IS relative to the TS in aqueous ethanol, which results in a slower reaction with a more product-like TS. Hammond and anti-Hammond effects on the TS arising from better stabilization of the IS over the TS dominate over the effects of increased nucleophile and LG basicity in determining the looser TS structure in aqueous ethanol. An altered TS structure is consistent with an altered reaction potential energy surface, in this case caused by a change in solvent polarity.


 Please wait while we load your content...
Please wait while we load your content...