Appraisal of the greenness profile of a chromatographic method for the simultaneous estimation of carbamazepine and oxcarbazepine, along with two potential impurities and three formulation excipients
Abstract
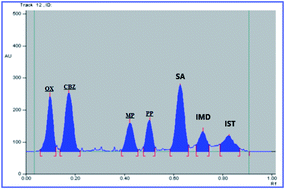
Structurally related carbamazepine (CBZ) and oxcarbazepine (OX) are two of the most commonly used antipsychotic drugs. The main impurities of CBZ, as described in both the USP and the BP, are iminodibenzyl (IMD) and iminostilbene (IST). Meanwhile, for non-pharmacopeial OX, the declared impurities include CBZ and IST. Prescribed oral suspensions of CBZ and OX contain additives including methyl paraben (MP), propyl paraben (PP) and sorbic acid (SA) as preservatives. An HPTLC method was introduced and developed for resolving the interference between CBZ, OX, their impurities, and the suspension additives in a single run, in addition to their quantitation with a high sensitivity that satisfies the USP requirements for the detection and quantitation of drug impurities. In the developed HPTLC method, CBZ and OX were measured in the range of 40–4000 ng per band, while IMD, IST, MP, PP and SA were in the range of 20–2000 ng per band, using a mixture of hexane : ethylacetate : formic acid : acetic acid (8 : 2 : 0.5 : 0.3, by volume) and UV scanning at 254 nm. The greenness profile of the method was evaluated by two different tools, the analytical Eco-Scale and the Green Analytical Procedure Index (GAPI), then a comparison between their results was conducted. This is the first time that the studied drugs, along with their impurities and suspension additives, were analyzed by a HPTLC method in a single run and within the limits required by the USP guidelines.


 Please wait while we load your content...
Please wait while we load your content...