Synthesis and spectral characterization of the first fluorescein-tagged iron(ii) clathrochelates, their supramolecular interactions with globular proteins, and cellular uptake†
Abstract
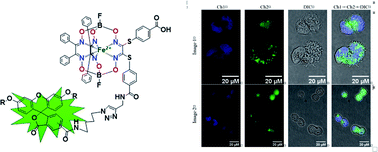
A fluorescein-tagged iron(II) cage complex was obtained in a moderate total yield using a two-step synthetic procedure starting from its propargylamine-containing clathrochelate precursor. An 11-fold decrease in fluorescence quantum yield is observed in passing from the given fluorescein-based dye to its clathrochelate derivative. An excitation energy transfer from the terminal fluorescent group of the macrobicyclic molecule to its quasiaromatic highly π-conjugated clathrochelate framework can explain this effect. The kinetics of the hydrolysis of the acetyl groups of acetylated fluorescein azide and its clathrochelate derivative in the presence of one equivalent of BSA evidenced no strong supramolecular host–guest interactions between BSA and the tested compounds. Study of a chemical stability of the deacetylated iron(II) clathrochelate suggested the formation of a supramolecular 1 : 1 BSA–clathrochelate assembly. Moreover, an addition of BSA or HSA to its solution caused the appearance of strong clathrochelate-based ICD outputs. The fluorescence emission anisotropy studies also evidenced the supramolecular binding of the fluorescein-tagged iron(II) clathrochelate to the BSA macromolecule, leading to a high increase in this type of anisotropy. Subcellular uptake of the fluorescein-tagged molecules was visualized using fluorescence microscopy and showed its distribution to be mainly in the cytosol without entering the nucleus or accumulating in any other organelle. An X-rayed crystal of the above propargylamide macrobicyclic precursor with a reactive terminal C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond contains the clathrochelate molecules of two types, A and B. The encapsulated iron(II) ion in these molecules is situated in the center of its FeN6-coordination polyhedron, the geometry of which is intermediate between a trigonal prism (TP) and a trigonal antiprism (TAP). The Fe–N distances vary from 1.8754(6) to 1.9286(4) Å and the heights h of their distorted TP–TAP polyhedra are very similar (2.30 and 2.31 Å); their values of φ are equal to 25.3 and 26.6°. In this crystal, the molecules of types A and B participate in different types of hydrogen bonding, giving H-bonded clathrochelate tetramers through their carboxylic and amide groups, respectively; these tetramers are connected to H-bonded chains.
C bond contains the clathrochelate molecules of two types, A and B. The encapsulated iron(II) ion in these molecules is situated in the center of its FeN6-coordination polyhedron, the geometry of which is intermediate between a trigonal prism (TP) and a trigonal antiprism (TAP). The Fe–N distances vary from 1.8754(6) to 1.9286(4) Å and the heights h of their distorted TP–TAP polyhedra are very similar (2.30 and 2.31 Å); their values of φ are equal to 25.3 and 26.6°. In this crystal, the molecules of types A and B participate in different types of hydrogen bonding, giving H-bonded clathrochelate tetramers through their carboxylic and amide groups, respectively; these tetramers are connected to H-bonded chains.


 Please wait while we load your content...
Please wait while we load your content...