Two methods for the preparation of sitagliptin phosphate via chemical resolution and asymmetric hydrogenation†
Abstract
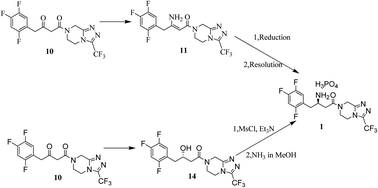
Two effective processes have been developed for the preparation of sitagliptin phosphate. The approach of chemical resolution obtained R-sitagliptin in five steps from commercially available starting materials using the inexpensive NaBH4 to reduce the enamine and then using (−)-di-p-toluoyl-L-tartaric acid to resolve racemates in 11% yield overall. The route successfully avoids the use of expensive noble metal as catalysts compared with traditional synthesis methods, resulting in greatly reduced costs and simplified synthetic routes. Other alternative asymmetric hydrogenation of β-ketomide routes for the synthesis of sitagliptin were found, two of the intermediates were synthesized for the first time.


 Please wait while we load your content...
Please wait while we load your content...