Niobium- and zirconium-catalyzed reactions of substituted 2 alkynylamines with Et2Zn†
Abstract
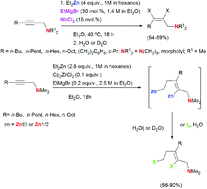
The NbCl5–EtMgBr-catalyzed reaction of N,N-disubstituted 2-alkynylamines with Et2Zn followed by hydrolysis or deuterolysis affords (2Z)-alkenylamines (reduction products of alkyne) in high yields. The reaction of N,N-disubstituted 2-alkynylamines with Et2Zn catalyzed by the Cp2ZrCl2–EtMgBr system occurs as 2-zincoethylzincation, resulting, after deuterolysis or iodinolysis, in the regio- and stereoselective formation of the corresponding dideuterated and diiodinated 2-alkenylamine derivatives with a trisubstituted double bond. This study demonstrates the difference between the catalytic effects of NbCl5 and Cp2ZrCl2 on the pathway of reaction of tertiary 2-alkynylamines with Et2Zn in the presence of catalytic amounts of EtMgBr.


 Please wait while we load your content...
Please wait while we load your content...