Considering both small and large scale motions of vascular endothelial growth factor (VEGF) is crucial for reliably predicting its binding affinities to DNA aptamers†
Abstract
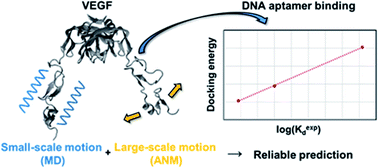
Vascular endothelial growth factor 165 (VEGF165), a predominant isoform of VEGF signal proteins, is an ideal target for developing drugs against various diseases. It is composed of a heparin binding domain (HBD) and a receptor binding domain (RBD), which are connected by a flexible linker. Among the two domains, RBD is utilized in binding with the signal transduction protein, the VEGF receptor (VEGFR). None the less for its pharmaceutical importance, structure-based studies for developing drugs has been severely hindered by the lack of its whole structure determination, mainly owing to the existence of the flexible linker. Fortunately, the utilization of computer simulation methods can offer a possibility to circumvent this difficult issue. Here, we employ ensemble docking in combination with the anisotropic network model analysis to examine the interactions between DNA aptamers and VEGF165. We model three-dimensional structures of aptamer variants based on their sequence information and perform docking calculations with the whole VEGF165 structure. Indeed, we show that we can closely reproduce the experimental binding affinity order among different DNA aptamer variants by inclusively considering the flexible nature of VEGF. In addition, we address how DNA aptamer that binds to HBD of VEGF165 impedes the interaction between VEGFR and VEGF165 through RBD, even though HBD and RBD are rather distant. The present study illustrates that the flexible docking scheme employed here can be applied to tricky cases that involve flexible proteins with undetermined structures, toward effectively predicting ligand binding affinities to such proteins.


 Please wait while we load your content...
Please wait while we load your content...